
Man-Po LEE
Adverse effects are common in patients taking antiretroviral agents. Of the over 1,000 patients who had received potent antiretroviral treatment in the Swiss HIV Cohort study, 47% and 27% of patients developed clinical and laboratory adverse events respectively.1 Adverse effects or drug toxicities are in fact the most common reasons for switching or discontinuing antiretroviral therapy.
Many factors predispose HIV-infected patients to adverse effects. Firstly, HIV is becoming a chronic disease while antiretroviral agents are used for longer periods. Long-term toxicities are increasingly recognised, which include such conditions as lipodystrophy and hyperlipidaemia. Secondly, HIV patients may have co-morbid conditions or diseases (e.g. alcoholism, hepatitis B or hepatitis co-infection may increase the risk of hepatotoxicity). Combination and concurrent medications may result in overlapping toxicities, for example, the use of stavudine (d4T) with didanosine (ddI), and zidovudine (ZDV) with ribavirin, while drug interactions may lead to dose-related toxicities. Furthermore, HIV-infected patients have systemic glutathione deficiency and reduced capacity to scavenge toxic metabolites.
Although the primary goal of antiretroviral therapy is potent and durable viral suppression, it is equally important to select a regimen that is best tolerated with minimal toxicities. This chapter focuses on the pathogenesis, clinical features, and management of some major toxicities associated with antiretroviral agents, including hepatotoxicity, skin rash, lactic acidosis, lipodystrophy, hyperlipidaemia, insulin resistance and diabetes. An overview of the spectrum of adverse effects is in Box 14.1.


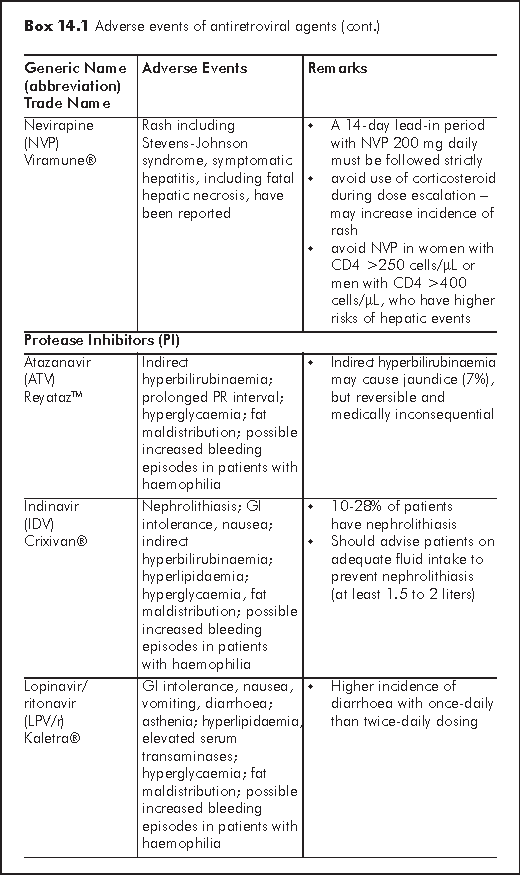
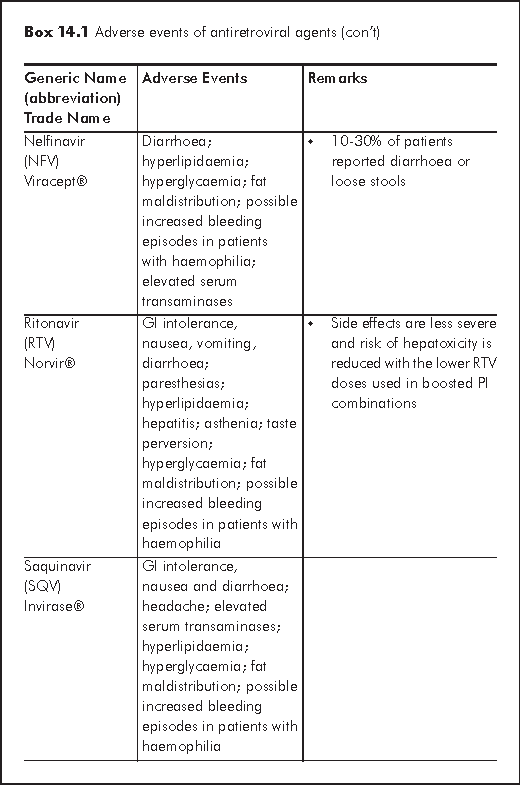
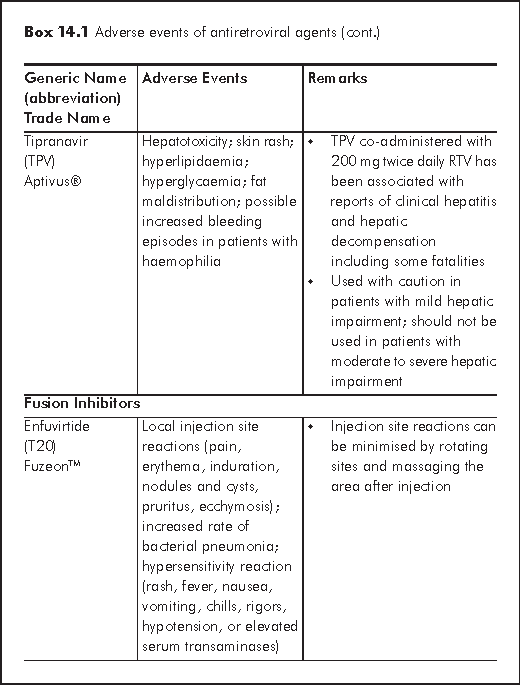
There are several mechanisms for hepatotoxicity relating to antiretroviral agents: hypersensitivity hepatitis (nevirapine or NVP), mitochondrial toxicity and hepatic steatosis (d4T, ZDV, ddI), direct hepatotoxicity (Protease inhibitors or PIs) and immune reconstitution in HCV or HBV co-infected patients.2 Factors that further increase risk of hepatotoxicity include HBV/HCV co-infection, alcoholism, substance abuse, abnormal baseline liver function. Regarding individual antiretroviral agent, female sex and high CD4 cell counts are associated with increased risk of NVP hypersensitivity hepatitis. Boosted Tipranvir with ritonavir (TPV/r) is more hepatotoxic than other PIs. Several nucleoside reverse transcriptase inhibitors (NRTIs) may cause hepatic steatosis (d4T >AZT, ddI). HBV flare may occur upon withdrawal of antiretrovirals - lamivudine (3TC), emtricitabine (FTC) or tenofovir (TDF).
NVP should be avoided in female with CD4 cell counts >250 cell/μL and male with CD4 cell counts >400 cell/μL. A 2-week dose escalation of NVP may reduce the incidence of hepatic events. TPV/r is contraindicated for patients with moderate to severe hepatic insufficiency. It is essential to monitor liver function regularly after initiating HAART (highly active antiretroviral therapy). The regimen may need to be discontinued if a patient is symptomatic or alanine aminotransferase (ALT) >5-10 x upper limit of normal (ULN). A new regimen is then reconstructed when symptoms have subsided and serum transaminases returned to normal.
Skin rash occurs more commonly with non-nucleoside reverse transcriptase inhibitors (NNRTIs), abacavir (ABC, an NRTI), and tipranavir (PI) than other NRTIs or PIs. Many studies have shown that rash is a common adverse event associated with NVP (all grades 17%, grade 3-4 around 6-8%), while less so with efavirenz (EFV) (all grades 10%, grade 3-4 less than 1%). In the 2NN study, skin rash, which led to treatment discontinuation, was observed in 6-12% of patients receiving NVP, and 4% of patients receiving EFV.3 A local series also reported high incidence of NVP-associated rash in HIV-infected Chinese.4 Patients taking ABC may develop hypersensitivity reaction presenting with acute onset of fever, diffuse skin rash, constitutional and respiratory symptoms (in descending order of frequency). The prevalence is around 5% in retrospective analysis.
The pathogenesis of drug hypersensitivity reaction is unknown. Suggested causes and mechanisms include immune-mediated responses, altered drug metabolism associated with glutathione deficiency or slow acetylator phenotype, co-existing infections with cytomegalovirus (CMV) or Epstein-Barr virus (EBV), or cytokines involvement.5
Most rashes are mild to moderate in degree, which typically manifest as diffuse maculopapular rash with or without pruritus. About 50% of mild to moderate degree rash resolves spontaneously with continuation of therapy. Therapy should be suspended if rash progresses to severe degree (presence of mucosal involvement, blistering, exfoliation or oedema) and/or if there is presence of high fever or hepatic dysfunction. A 14-day lead-in period with NVP 200 mg daily must be followed strictly in order to minimise the occurrence of skin rash. Corticosteroids are ineffective in preventing NVP hypersensitivity and may even increase incidence of skin rash. Rechallenge may be possible for mild to moderate NNRTI rash. Rechallenge with ABC should however, never be pursued after a hypersensitivity reaction.
Lactic acidosis is attributed to mitochondrial DNA gamma polymerase inhibition, resulting in impaired synthesis of mitochondrial enzymes that generate ATP. It is caused by NRTI use which occurs at different frequencies (d4T > AZT, ddI > 3TC, FTC, ABC, TDF). Other risk factors include female gender, obesity and pregnancy. Patients usually present with gradual onset of nausea, vomiting, abdominal pain, weight loss and fatigue. As toxicity progresses, patients may develop dyspnoea, muscular weakness, organ failure, cardiovascular collapse and death.6,7 Mortality is high (20-60% with lactate >10 mmol/L). Diagnosis is confirmed by serum lactate >2 mmol/L (serum blood drawn without tourniquet) and severity is graded accordingly (mild 2-5 mmol/L, moderate 5-10 mmol/L, severe >10 mmol/L). Venous blood should be drawn without tourniquet. Other abnormal laboratory tests include low bicarbonate, increased anion gap, raised CPK, LDH, serum transaminases, amylase and lipase.
Antiretroviral agents should be discontinued for symptomatic patients. Treatment is mainly supportive. Severe cases may require IV bicarbonate infusion, dialysis and mechanical ventilation. There are anecdotal reports of the use of thiamine, riboflavin, L-carnitine and vitamin C. Antiretroviral treatment options after resolution of lactic acidosis include use of NRTIs with less propensity of mitochondrial toxicity (ABC, TDF, 3TC, FTC) or use of NRTI-sparing regimens (PI + NNRTI). Serum lactate should be closely monitored after restarting NRTIs.
The exact pathogenesis of lipodystrophy remains unknown. Two processes are recognised, lipoatrophy and fat accumulation. Lipoatrophy involves peripheral fat loss in face, proximal extremities and buttocks. It is caused by mitochondrial toxicity secondary to NRTIs, notably d4T. Fat accumulation may manifest as neck or dorsocervical fat pad (buffalo hump), increased abdominal girth and breast size. It is multifactorial and is associated with PI use. Both lipoatrophy and fat accumulation can be associated with hyperlipidaemia or insulin resistance. Other risk factors include low baseline Body Mass Index (BMI) and total duration of HAART. Reported frequencies of lipodystrophy are high.8
There is as yet no accurate and convenient diagnostic method, and diagnosis remains clinical.9 In clinical trials, measures like DEXA, MRI and CT have been used. There is no proven therapy for lipodystrophy. Switching to other antiretroviral agents may slow progression or possibly reverse effects (e.g. switching d4T to TDF, ABC, 3TC or FTC, or eliminating PI when safe). Metformin, rosiglitazone and recombinant human growth hormone have been used with variable efficacy. Injectable polylactic acid has reportedly been used in some series for treatment of severe facial lipoatrophy.
The frequency and pattern of hyperlipidaemia vary with different antiretroviral agents. Between 47-75% of patients receiving PI developed hyperlipidaemia. All PIs except atazanavir (ATV) may give an elevation of total cholesterol, LDL and TG and decrease in HDL, the risk of which is highest for ritonavir (RTV) and kaletra (LPV/r). The use of d4T stands the highest risk among NRTIs for TG and LDL elevation; but low or no risk with TDF and ABC. NNRTIs use may increase HDL and TG. Disturbance of lipid profile usually begins in weeks to months after starting therapy. Hyperlipidaemia is an important risk factor for cardiovascular disease. Use of antiretroviral agents is associated with a 26% annual increase in myocardial infarctions and other cardiovascular and cerebral vascular events.10
ACTG has prepared guidelines for the evaluation and management of hyperlipidaemia in HIV-infected patients receiving HAART. Before deciding treatment, it is important to assess coronary heart disease (CHD) risk using a 2-step procedure (see Algorithm 14(A)). First, the number of risk factors is counted (cigarette smoking, hypertension, low HDL cholesterol, family history of premature CHD, >45 years for men and >55 years for women). Second, for persons with >=2 risk factors, 10-year CHD risk is estimated using Framingham scoring to identify individuals who warrant intensive treatment. Lifestyle modification such as smoking cessation, healthy diet and exercise should be stressed in all patients with hyperlipidaemia. One may consider switching to antiretroviral agents with lower propensity for causing hyperlipidaemia provided that viral control is not jeopardised. Although in a randomised trial comparing substitution of NNRTI for a protease inhibitor versus lipid-lowering therapy, statin or fibrate were significantly more effective than switching.11 Pharmacological agents include HMG-CoA reductase inhibitors (statins) and fibric acids. Statins are potent agents to lower total cholesterol and LDL. It is advised to use statins that have less drug interaction with antiretroviral agents (e.g. pravastatin, rosuvastatin, fluvastatin or atorvastatin). Fibric acids are preferred for predominant high TG (e.g. gemfibrozil or fenofibrate).12,13 Hyperlipidaemia in general in people living with HIV/AIDS is also discussed in Chapter 17 in primary care context.
Insulin resistance in PI-treated patients is common. Besides, up to 3-5% of patients developed diabetes. Risk factors for the development of insulin resistance or DM include lipodystrophy, family history of DM, obesity, age, hepatitis C, and low nadir CD4 count. Lipodystrophy can cause insulin resistance by impairing insulin action and inducing defects in beta-cell function.14 Use of LPV/r, indinavir (IDV) and RTV have shown to be associated with insulin resistance while ATV does not. Elevation of blood glucose usually begins in weeks or months after beginning therapy. Fasting glucose should be monitored regularly after HAART.
Patients with insulin resistance or DM should be advised on dietary control, regular exercise and coronary risk factors modification (such as smoking cessation and lipid control). Drug therapy include metformin, thiazolidinediones (rosiglitazone and pioglitazone) or sulfonylureas. Metformin and thiazolidinedione have additional advantage of improving insulin resistance and decreasing visceral fat accumulation.15 Insulin is used if oral hypoglycaemic agents fail. One may also consider switching from PI-based to NNRTI-based regimen provided that viral control is not jeopardised.
Fellay J, Boubaker K, Ledergerber B, et al. Prevalence of adverse events associated with potent antiretroviral treatment: Swiss HIV Cohort Study. Lancet 2001;358:1322-7.
Nunez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol 2006;44(1 Suppl):S132-9.
van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004;363:1253-63.
Ho TT, Wong KH, Chan KC, Lee SS. High incidence of nevirapine-associated rash in HIV-infected Chinese. AIDS 1998;12:2082-3.
Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet 2000;356:1423-30.
Carr A. Lactic acidemia in infection with human immunodeficiency virus. Clin Infect Dis 2003;36(Suppl 2):S96-S100.
Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis 2003;3:329-37.
Tien PC, Grunfeld C. What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis 2004;17:27-32.
Carr A, Emery S, Law M, et al. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet 2003;361:726-35.
Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993-2003.
Calza L, Manfredi R, Colangeli V, et al. Substitution of nevirapine or efavirenz for protease inhibitor versus lipid-lowering therapy for the management of dyslipidaemia. AIDS 2005;19:1051-8.
Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:613-27.
Schambelan M, Benson CA, Carr A, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr 2002;31:257-75.
Andersen O, Haugaard SB, Andersen UB, et al. Lipodystrophy in human immunodeficiency virus patients impairs insulin action and induces defects in beta-cell function. Metabolism 2003;52:1343-53.
Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA 2000;284:472-7.