Nelson LS LEE, Eric CC LEUNG, CM TAM
Mycobacterium tuberculosis (MTB) infection has re-emerged together with two new accompanying problems, that of drug resistance and HIV co-infection. In 1993, the World Health Organization (WHO) declared TB as a global emergency, calling for international collaboration in the fight against the disease. In 2005, WHO estimated that a third of the world's population is infected with MTB; and more than eight million people acquired MTB infection annually. It was estimated that two million people died from MTB infection every year (WHO TB Programme). In fact, both TB and HIV are endemic in many developing Asia and African countries.
In Hong Kong, the notification rate of TB decreased from a peak of 697.2 per 100,000 in 1952 to around 100.9 in 1995, and thereafter the situation has become more or less static. In 2006, the total number of notified cases is 5856, equivalent to a rate of 85.4 per 100,000 (provisional figures).1 Hong Kong is classified as a place of intermediate TB burden with good health infrastructure in the Western Pacific Region. At present, HIV-related TB cases represent only a minority of the annual TB notification. Unlinked anonymous testing has shown that less than 1% of TB patients in the chest clinics were HIV seropositive.2 There has, nevertheless, been a slow rising trend over the years; one explanation being the increasing cumulative number of HIV-infected persons developing AIDS with the passage of time. In Hong Kong, both extrapulmonary TB (at any CD4 count) and pulmonary TB (in those with a CD4 count below 200/μL) are AIDS-defining conditions for surveillance purpose. Between 1996 and 2005, a total of 277 TB-HIV co-infected cases were recorded in a registry maintained by the Department of Health. During the same period, 167 of 607 AIDS cases had TB as the primary AIDS defining illness.1
Although MTB infects both HIV-positive and negative individuals, the risk of progression to active TB in those co-infected with MTB (latent TB) and HIV is increased 100-fold, with a rate of around 5% to 10% per year.3,4 The second commonest mechanism is primary MTB infection, the proportion of which may vary across populations. HIV predisposes to MTB infection at all ranges of CD4 cell counts, but the clinical features may vary according to the degree of immunosuppression. With a CD4 cell count >350/μL, lung lesions appear to be more typical with upper lobe infiltrates and the development of cavities. With lower CD4 cell counts (e.g. <50/μL), extrapulmonary TB (± concurrent pulmonary TB) is more common, accounting for up to 40-80% of the manifestations. Patients may present with lymphadenitis, pleuritis, pericarditis, meningitis, CNS tuberculomas, and even disseminated diseases. Chest radiographs may show atypical features like lower lobe or middle lobe diseases, miliary infiltrates; cavitations may not occur. It is noted that a transient decrease in CD4 cell count and a 5 to 160-fold rise in viral load have been demonstrated in MTB infections; and MTB infection is associated with more rapid progression of HIV infection.5,6
Diagnosis of TB in HIV-infected patients demands a high index of suspicion because of the often 'atypical' clinical features. A detailed medical history and physical examination is essential. For most patients, the initial investigations include a chest radiograph and sputa examinations (see below). Even for patients with unremarkable chest radiology findings, sputum examination for acid fast bacilli (AFB) may still be indicated if the clinical features/presentation is compatible with TB. Other clinical samples for microbiological diagnosis include bronchoaveolar lavage, transbronchial biopsy, pleural fluid, early morning urine, lymph node aspirate, pericardial fluid, cerebrospinal fluid, bone marrow biopsy, and synovial fluid, whenever clinically indicated. Imaging studies like ultrasound or CT scans may be used to localise the disease and guide biopsies. Patient with infectious TB per se is not an indication for hospital admission. However, if an inpatient is suspected of having infectious TB, the physician should assess the need for respiratory isolation. This can be discontinued when there is clinical and radiological response to treatment, and after 3 consecutive negative smears (collected on different days) have been obtained.3 Although a range of laboratory diagnostic tests are available, the basic tests include strain identification and drug susceptibility test for positive culture isolates apart from direct microscopy and culture.
The standard test for pulmonary TB is morning expectorated sputa for 3 days for AFB smear and culture.2 The gold standard of diagnosing MTB infection is culture of the organism. It is also the most sensitive diagnostic method, requiring only 10-100 viable organisms per ml to become positive. In Hong Kong, this is routinely followed by drug susceptibility testing, which is essential for individual treatment and monitoring the trend of resistance development in the community. Drug susceptibility testing is a key component of the "DOTS-Plus" strategy. However it may take 4-10 weeks to yield a positive mycobacterial growth. On the other hand, a sputum sample must contain 5000-10000 bacilli/mL to yield a positive AFB smear. Although the test is simple, rapid, readily available and inexpensive, sensitivity of AFB smear is around 50% (22-78%), which is similar for patients with and without HIV/AIDS. Its sensitivity is higher in cavitary disease. A positive sputum smear also indicates significant infectivity in untreated patients, which implies the need for respiratory isolation where appropriate. Induced sputa and bronchoscopy can be considered if there is no sputum production. Specificity of AFB smear is affected by the prevalence of non-tuberculous mycobacterial in particular MAC infections, but most patients with positive AFB smears of respiratory specimens in patients with HIV/AIDS and compatible symptoms normally have MTB infection, and should be investigated as such. For miliary TB, sputum cultures are positive in only 25%, but multiple other specimens are either AFB smear or culture positive (including blood) in 50-60% of the cases.
To expedite the diagnosis of TB and determination of its susceptibility, the use of BACTEC system is recommended in situations where culture confirmation and sensitivity pattern are important in the clinical management. The radiometric liquid (broth) BACTEC system reduces average detection time to 10-14 days. Susceptibility results to commonly used drugs can follow shortly. The radiometric method is usually semi-automated and involves handling of radioactive materials. A fully automatic non-radiometric system is perhaps more advantageous. These systems have been shown to be comparable to conventional agar method in accuracy and reliability. High running costs associated with these test formats are the usual considerations that hamper more extensive use.
Nucleic acid amplification (NAT) tests are more sensitive than AFB smear (80%). It is also highly specific (98%) for MTB infection. It is 95% sensitive for AFB smear positive cases, and allows rapid diagnosis of TB. However, its sensitivity is lower (48-53%) in smear negative, but culture positive cases. The current recommendation is that they may be used with smear positive cases or smear negative cases with high index of suspicion (on respiratory specimens). The primary determinant of successful NAT testing for tuberculosis is the shedding of mycobacterial DNA in secretions from caseating granulomas and its dissemination to sterile body fluids or tissue biopsies. In multibacillary diseases with a high mycobacterial load, a positive Ziehl-Neelsen smear with a positive NAT is diagnostic of active tuberculosis, whereas a positive Ziehl-Neelsen smear with a negative NAT in the absence of inhibitors would indicate non-tuberculous mycobacterial disease. The role of NAT is more important in paucibacillary diseases with low mycobacterial loads. The application of nucleic acid amplification test may also be extended to other non-respiratory specimens. Studies have shown that similar techniques can be applied to cerebrospinal fluid, urine, gastric aspirate, liver biopsy and bone marrow specimens with encouraging results. The presence of polymerase chain reaction (PCR) inhibitors, however, especially in extrapulmonary specimens, may produce false-negative results. In a systemic review,4 the sensitivity of PCR in pleural fluid and CSF was quite low (pleural fluid 27.3 to 81% and CSF 21-56%). In a local study of PCR as diagnostic aid,7 using culture as the gold standard, the overall sensitivity of TB PCR was 78.3%, and for pulmonary and extrapulmonary specimens it was 82.3% and 72.0%, respectively. Finally, it should be emphasised that NAT cannot differentiate between dead and live tubercle bacilli, and will not provide drug susceptibility pattern which is essential to guide management.
MTB strain typing by DNA fingerprinting (restriction fragment length polymorphism, RFLP) using insertion sequence IS6110 as the standard probe has been proven to be a powerful molecular tool for epidemiological investigation, especially in outbreak situations. Rapid detection of drug resistance is particularly useful when there is a high chance of multidrug-resistant TB (MDR-TB) as in the cases of treatment failure or early relapse after treatment completion. Currently genetic methods are most efficient in the diagnosis of rifampicin (RIF) resistance, because about 96% of the resistant isolates have a mutated rpoB gene.8 Testing for RIF resistance by the detection of mutations of the MTB rpoB gene has been recommended because of its high predictive value for multidrug-resistance and its technological feasibility. Direct detection of RIF resistant MTB in IS6110 PCR or PCR positive respiratory specimens by rpoB PCR-DNA sequencing of the 81-bp RIF resistance determining region was found to be feasible in a local study.9 In contrast to RIF, genotypic testing of resistance to INH is more difficult. Alterations in at least four genes - katG, inhA, ahpC, and kasA - are associated with INH resistance, and the mutations may occur in multiple sites.10
Anti-tuberculous therapy and highly active antiretroviral therapy (HAART) initiated at the same time can lead to problems of overlapping drug toxicities, drug-drug interactions (esp. with RIF), non-adherence and sometimes immune reconstitution disease. It is generally recommended that antituberculous therapy be initiated first, followed by HAART 4-8 weeks later. The possible exception is patients with advanced HIV with CD4 counts <50/μL. According to the WHO, for patients with CD4 count <200/μL, physicians may start ART 4-8 weeks after anti-tuberculous treatment with an efavirenz (EFV)-based HAART regime. The alternatives include: boosted saquinavir (SQV/r), abacavir (ABC) or nevirpaine (NVP). For patients with CD4 counts in the range of 200-250/μL, similar ART regimes can be considered after initial antituberculous treatment. For the group of patients with CD4 cell count >350/μL, deferring HAART is recommended. When starting PI or NNRTI other than EFV, physicians should substitute rifabutin (RFB) 2 weeks prior to PI or NNRTI to provide 'wash out' for RIF. An outline of TB management is described in the Algorithm 27(A).
If supervised properly, standard anti-TB regimens are as effective in HIV positive as in negative patients. However, some studies showed higher relapse rates with a 6-month regime when compared to longer ones. Modification of drugs and prolongation of treatment course will be necessary in the face of drug resistance, drug interaction, CNS disease (and possibly joint and bone TB), or unsatisfactory clinical responses. Notably the occurrence of MDR-TB (as defined by isoniazid (INH) and RIF resistance), and TB outbreaks have been linked to non-adherence. For these reasons, DOT (directly observed treatment) is deemed the standard of care. DOT could be provided daily or intermittently (e.g. three times a week). In Hong Kong an infrastructure of expertise on DOT is in place through the network of chest clinics in the public service. It is to the patient's advantage that this be fully utilised, especially in the face of drug resistance and doubtful adherence.2
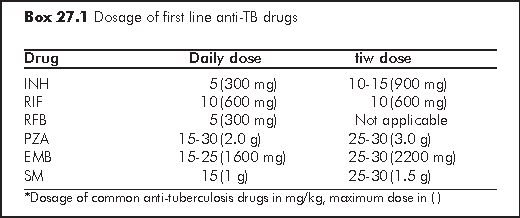
INH and RIF are the two most potent, bactericidal antituberculous agents which form the cornerstone of treatment. The optimum TB treatment is one containing a rifamycin. A non-rifamycin anti-TB regimen is called for when: (a) ART regimen incompatible with RFB and RIF is needed for control of HIV, or (b) there is RIF resistance (N.B. only one third of RIF-resistant TB remains susceptible to RFB). A non-rifamycin-containing regimen was associated with lower survival and higher recurrence rate. Every effort should be made to use a rifamycin-based regimen for the entire course of therapy in HIV-TB patients.11,12
In HIV-infected patients in Hong Kong, the standard regimen for pulmonary TB is one containing INH and a rifamycin (RIF) for 9 months, or at least 4 months after culture conversion to negative.2 Pyrazinamide (PZA) and ethambutol (EMB) / streptomycin (SM) are also added in initial phase (first 8 weeks). A 9-month regime is important to reduce rate of relapse especially among patients presenting with cavitary disease, poor initial clinical responses, and among those with persistently positive cultures at 2 months. Delayed culture conversion and resolution of signs and symptoms by 2 month should also call for an evaluation for drug resistance, malabsorption, and possible drug-drug interactions. For extrapulmonary TB, including bone and joint TB, a minimum of 9 months is required. For CNS involvement, treatment should be prolonged to 12 months. For patients with CD4 cell count below 100/μL, the initial phase (first 8 weeks) should comprise of a daily regime; and in the continuation phase, treatment should be daily or at least thrice per week.
Cohort studies and clinical trials suggest that patients with advanced HIV/TB disease are at increased risk of treatment failure and relapse.
These studies also demonstrated that a high percentage of treatment failure and relapse had acquired rifamycin resistance. It appears to be related to intermittent dosing regimen (once or twice weekly therapy) and advanced HIV disease CD4 <100/μL. Given the consistency of the association between acquired rifamycin resistance and highly intermittent dosing, patients with advanced HIV disease (CD4 cell count <100/μL) should not be treated with intermittent twice weekly regimens13 (Note: In practice, anti-TB drugs are not prescribed at a frequency of less than thrice per week in the public service in Hong Kong.) The anti-tuberculous regime may need to be modified in view of potential drug-drug interactions and overlapping toxicities with HAART. Using non-rifamycin-based regimens may avoid drug-drug interactions but at the cost of necessitating extended treatment duration, and likely treatment efficacy. Thus the best approach should be tailored to the circumstances of each individual case.

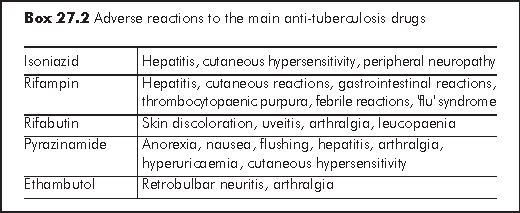
Reactions to anti-TB drugs are more common with HIV positive individuals in whom life threatening adverse events may occur. Careful clinical monitoring is necessary (e.g. hepatitic symptom, skin rash).2 Pretreatment and serial assessments of liver function and regular visual assessment (with EMB) may be indicated. In particular, chronic hepatitis B patients have higher chance to develop drug-related hepatotoxicity. Pyridoxine supplementation may be necessary with INH to prevent peripheral neuropathy. Desensitisation and/or rechallenge in the event of sensitivity or toxicity are complicated and are best dealt with by experts in this field. Despite the differences, the treatment response is largely the same in patients with or without HIV/AIDS. Sputum culture becomes negative <=2 months in 85% after treatment in drug susceptible cases. Persistence of positive cultures at >=4 months on the other hand may indicate non-compliance or drug resistance. Physicians also need to distinguish immune reconstitution disease from treatment failure. Drug compliance can be cross-checked by urine testing for INH metabolite and serum assay for RIF level. Repeated imaging may sometimes be indicated to document resolution of disease.
After initial clinical improvement, paradoxical worsening of disease develops in up to 36% of patients treated with concurrent anti-tuberculosis treatment and HAART, as soon as 2 weeks after initiation of the latter. The reaction is characterised by fever, worsening of chest infiltrates on radiograph, development of peripheral and/or mediastinal lymphadenopathy, expanding CNS lesions, and large pleural effusions. In contrast, these paradoxical reactions occur in 7% of those initiated on anti-tuberculous treatment only without HAART. Such reactions are associated with partial restoration of immune function after starting HAART or anti-TB treatment, are not associated with dissemination of disease or reversion of smear/culture-negative status to a positive status. The differential diagnoses are drug fever, treatment failure, drug resistance, other opportunistic infections and lymphoma. Almost invariably a paradoxical reaction is associated with a concurrent drop of viral load and a temporal relationship with HAART or anti-TB treatment in HIV-infected persons, and therefore provides the hint.
These paradoxical reactions can sometimes be quite severe, leading to significant morbidity (Chapter 15). Treatment with corticosteroids may be indicated in such circumstances. For severe reactions, prednisolone 1 mg/kg/d for 1-2 weeks can be given, then taper in the following weeks. In most situations, continuation with HAART and anti-tuberculous agents are recommended. For mild to moderate reaction, symptomatic treatment is adequate.
Since RIF strongly induces the CYP 3A component of cytochrome P450, it increases clearance of protease inhibitors (PI) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Conversely many PIs and NNRTIs are either inhibitors or inducers of the enzyme, thus affecting the level of rifamycins. The concurrent use of RIF with NNRTI or PI will have to be carefully monitored, if not contraindicated. Similarly, RIF reduces the concentration of fluconazole and itraconazole, and ketoconazole can inhibit the absorption of RIF. For the most updated information on drug-drug interactions between anti-tuberculosis and antiretroviral agents: Centers for Disease Control and Prevention (CDC) www.cdc.gov/nchstp/tb/TB_HIV_Drugs/TOC.htm
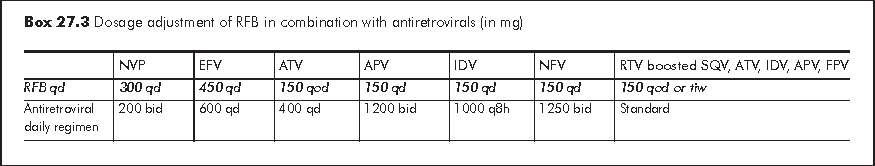
RFB, another rifamycin, has substantially less activity as an enzyme inducer. Its efficacy against MTB has been fairly well established in a few comparison trials with RIF. In the presence of PI or NNRTI, RFB is generally preferred to RIF because of complex drug-drug interactions with the latter. RIF is contraindicated with delarvirdine (no longer used), fosamprenavir (FPV), indinavir (IDV), nelfinavir (NFV), and saquinavir (SQV). In practice, the use of a RIF-based regimen in HIV-related TB is restricted to EFV-based HAART. NVP, and Kaletra (LPV/r) boosted with additional ritonavir (RTV) 300 mg bid, may also be considered. RFB in normal or reduced dosage can be used together with LPV/r), NFV, IDV, SQV/r, FPV/r, RTV, and atazanavir (ATV), while SQV alone is contraindicated (Box 27.3). With EFV, RFB will have to be increased to 450 mg daily, while with NVP the dose of RFB is unaltered. There is insufficient data to recommend RFB use with dual PI or PI/NNRTI combinations. Physician should monitor adverse effects of RFB (e.g. uveitis, skin discoloration) or reduced potency of PI when they are given in combination.
For those who are already on RIF and about to be switched to RFB, a washout period of 2 to 3 weeks is recommended after the discontinuation of RIF. In the meantime, RFB in full dosage i.e. 300 mg per day is given until the PI is started. Dosage of both RFB and ART may need to be adjusted. Conversely if a PI is to be discontinued so that RIF can be started (in general, this is not recommended), 2-3 days of washout are allowed. The RIF is then started at half dose and increased to full dose after a week.
Patients with drug resistant TB should be managed with great caution. Although individual situations vary, the following are the major principles in managing resistant TB: (a) DOT is mandatory; (b) A single drug is never added to a failing regimen; (c) The addition of drug should include at least 2-4 agents that are active in vitro.2
For monoresistant TB, the following are some suggested regimens for management:
SM resistance - use standard regimen;
INH resistance - RIF/RFB+PZA+EMB+SM for 2 months, then RIF/RFB+PZA+EMB for 10 months;
RIF resistance (uncommon, need to watch out for MDR-TB) - use INH+PZA+EMB+SM for 3-4 months, then INH+PZA+EMB for a total duration of 18 months; alternatively, treatment needs to be given for 12 months after negative culture;
PZA resistance (sensitivity result may not be reliable; also watch out for M. bovis which is naturally resistant) - use RIF+INH+EMB for 2 months, then RIF+INH for 7 months.
For MDR-TB (as defined by the presence of both INH and RIF resistance) patients with known susceptibility pattern, the treatment regimen should comprise 5-6 drugs to which the organism is or is likely to be susceptible for the initial 6 months, followed by 3-4 drugs subsequently. The inclusion of an injectable agent for the initial months and a fluoroquinolone all through are generally recommended. Daily regime should be used, except perhaps for the injectables. Drugs showing in vitro resistance are generally excluded, with the possible exception of use of isoniazid in cases of low level resistance. Apart from first-line anti-TB drugs, available drugs for treatment of MDR-TB include the fluoroquinolones (e.g. levofloxacin, ciprofloxacin, moxifloxacin), aminoglycosides (e.g. kanamycin, amikacin), prothionamide/ethionamide, cycloserine, para-aminosalicylic acid, and clofazimine. Drugs that have not been used to treat the patient before are preferred, and so are bactericidal drugs rather than bacteriostatic drugs. The total duration of therapy for MDR-TB has not been clearly established; most will recommend a total duration of 18 months at least, or 18 months after culture being converted negative. However, local experience suggests that, with combination drug treatment and the inclusion of fluoroquinolones to which the bacilli are still susceptible, the total duration may be shortened to 12 to 15 months, or one year after sputum culture conversion.
HIV positive patients with latent TB infections have high risks for TB reactivation, and therefore treatment is indicated. Latent TB infection is usually diagnosed by a positive tuberculin skin test (TST) as defined by an induration size >=5 mm, after ruling out active disease. Newer blood tests based on detection of IFN-γ released by T cells in response to M. tuberculosis - specific antigens may offer an alternative to the TST skin test.14 These blood tests have operational advantages over the skin test because no return visit is required, results are available by the next day, the reading of the results is operator independent and repeat testing does not cause boosting. Preliminary evidences suggest that some of these tests may be useful in HIV-infected patients, with a sensitivity of up to 92%.15
The preferred treatment regimen for latent TB infection in HIV-infected persons is INH 300 mg/day plus pyridoxine 10-50 mg/day for a total of 9 months (or INH 900 mg 2x/week plus pyridoxine for 9 months). The 2-month rifampin/pyrazinamide regime although effective, is associated with increased hepatotoxicity and must be used with caution together with close monitoring.16
Tuberculosis and Chest Service, Department of Health, Hong Kong SAR Government. Annual Report 2005.
Tuberculosis and Chest Service. Tuberculosis Manual 2006. Hong Kong: Department of Health, 2006. Available from www.info.gov.hk/tb_chest/doc/Tuberculosis_Manual2006.pdf
Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005;54:1-141.
Cheng VC, Yew WW, Yuen KY. Molecular diagnostics in tuberculosis. Eur J Clin Microbiol Infect Dis 2005;24:711-20.
Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 1999;340:367-73.
Munsiff SS, Alpert PL, Gourevitch MN, Chang CJ, Klein RS. Chang CJ, Klein RS. A prospective study of tuberculosis and HIV disease progression. J Acquir Immune Defic Syndr Hum Retrovirol 1998;19:361-6.
Cheng VC, Yam WC, Hung IF, et al. Clinical evaluation of the polymerase chain reaction for the rapid diagnosis of tuberculosis. J Clin Pathol 2004;57:281-5.
Marttila HJ, Soini H. Molecular detection of resistance to antituberculous therapy. Clin Lab Med 2003;23:823-41.
Yam WC, Tam CM, Leung CC, et al. Direct detection of rifampin-resistant mycobacterium tuberculosis in respiratory specimens by PCR-DNA sequencing. J Clin Microbiol 2004;42:4438-43.
Leung ET, Kam KM, Chiu A, et al. Detection of katG Ser315Thr substitution in respiratory specimens from patients with isoniazid-resistant Mycobacterium tuberculosis using PCR-RFLP. J Med Microbiol 2003;52(Pt 11):999-1003.
American Thoracic Society, CDC, and Infectious Diseases Society of America. Treatment of tuberculosis. MMWR 2003;52(RR11);1-77.
CDC. Updated guidelines for the use of rifamycins for the treatment of tuberculosis among HIV-infected patients taking protease inhibitors or non-nucleoside reverse transcriptase inhibitors. MMWR 2004 Version 1.20.04. Available from www.cdc.gov/nchstp/tb/tb_hiv_drugs/toc.htm
Centers for Disease Control and Prevention (CDC). Acquired rifamycin resistance in persons with advanced HIV disease being treated for active tuberculosis with intermittent rifamycin-based regimens. MMWR Morb Mortal Wkly Rep 2002;51:214-5.
Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-76.
Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 2002;16:2285-93.
Centers for Disease Control and Prevention (CDC); American Thoracic Society. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection--United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52:735-9.