Siu-Keung LAM, Mandy SF WONG, Lowina HY TSE
In Hong Kong, mother-to-child-transmission (MTCT) of HIV is the major cause of paediatric infection. Although the relative proportion of MTCT among all local HIV infection is small (19 reported cases of perinatal infection as of the end of 2006), it is the single most important route by which a child is infected with HIV, now that all donated blood is screened. MTCT is unique in the sense that a window of opportunity exists whereby appropriate biomedical intervention can prevent the occurrence of the infection. Furthermore, as HIV infected women are enjoying better general health while receiving highly active antiretroviral therapy (HAART), they may be more keen to start a family and rear a child, whereas in the past most would avoid or terminate pregnancy. This is also reflected in the local demand of infertility service from HIV infected couple.1 Against the background of rising number of infected women, the HIV positive pregnancy will likely be more common in the future.
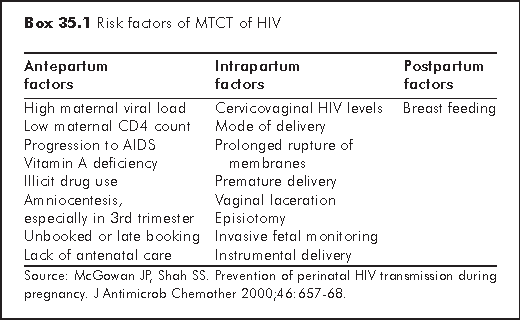
Many factors have been identified to be associated with increased risk of HIV transmission (Box 35.1). MTCT may occur during pregnancy, delivery and the postnatal period, though infection in early pregnancy is relatively infrequent.

HIV may cause placental infection and subsequently intrauterine infection of fetus. This possibility is supported by the findings that some placental cells express CD4, and the fact that two peaks of HIV positivity can be detected in the infected neonate: 38% of the HIV infected infants tested positive with HIV DNA PCR within 48 hours of life and almost all the rest at 2 weeks.2
Transmission during parturition may result from exchange of body fluids, including blood and genital secretions of the mother. This explains why babies born to mothers with membranes ruptured for more than 4 hours prior to delivery had a higher risk of infection (25% vs 14%).3 Ascending infection from the maternal genital tract may also be important as judged from the correlation between infection and the level of HIV in cervicovaginal fluids.4
Breastfeeding is the medium of transmission in the postnatal period. It accounts for the higher rate of MTCT in developing countries. In one randomised trial carried out in Kenya,5 the rate of breast milk transmission was 16.2% at 24 months (36.7% in breastfeeding arm vs 20.5% in control arm). In developing countries, the risk of infectious gastroenteritis as a result of the poorer access to clean water and medical resources may outweigh those due to paediatric HIV infections. In developed countries where safe formula feeding is available, breastfeeding should be avoided in HIV infected women.
There are various strategies for antenatal HIV testing: mandatory, voluntary (opt-in), universal (with opt-out), and epidemiological surveillance, the latter using such methodology as unlinked anonymous screening. The Department of Health reported a low prevalence of HIV antibody in neonatal cord blood from unlinked anonymous screening (0 to 0.033%) from 1997 to 2000. A study identified 41 HIV-exposed pregnancies of 32 women between 1992 and 1999 in Hong Kong. Out of the 12 HIV-related pregnancies diagnosed before delivery, only 1 baby was infected. This contrasted with those whose diagnosis was made late after delivery, where 9 out of 14 (64.3%) were infected - a relative risk of 8. The importance of early diagnosis is therefore clearly evident.
To address the problem of missed diagnosis, the Scientific Committee on AIDS in March 2000 proposed measures to decrease MTCT of HIV including the universal antenatal HIV testing. In the same year6 a local antenatal clinic reported an HIV prevalence of 0.05% in antenatal women and the high acceptance rate (97.5%) for routine HIV testing.
From September 2001, all antenatal patients booked in public antenatal clinics (Hospital Authority hospitals and Maternal & Child Health Clinics) are offered free HIV antibody test on top of their usual antenatal blood tests.7 Pregnant women are given relevant information and opportunity for opt-out. To promulgate the practice, similar guideline was published by Hong Kong College of Obstetricians and Gynaecologists in November 2001.8 Two reports have so far been released after the implementation of the universal antenatal HIV screening in 20039 and 200510 to determine the effectiveness of the programme. From September 2001 to December 2004, 132333 out of 136052 pregnant women attending public antenatal clinics have received the HIV test (97.26%). Twenty-eight women were found to be HIV positive in the same period. Ten pregnant women requested termination of pregnancy and 14 mothers received antiretroviral therapy. Fifteen babies were born locally and one baby was found to be infected (mother presented late). Prompt diagnosis and intervention is the key to effective MTCT prevention.
The use of rapid HIV test when the patient is near term or in labour is a useful supplemental procedure to improve coverage of the screening. It has also been the practice in some developing countries where there is no antenatal care nor screening. In Hong Kong there has been a decreasing trend of the proportion of deliveries with known maternal HIV status. When an expectant mother is admitted in late pregnancy, during or after delivery, she should receive a rapid HIV test with reliable sensitivity. A negative test effectively rules out HIV infection, while prophylaxis can be offered after a positive result, though confirmation is still required for a definitive diagnosis to be made. As explained below in the section on "Regimens in late presentation", there is still benefit to the infant in administration of antiretroviral therapy at this late gestation. The mother could also be counselled for other measures to decrease the chance of MTCT namely caesarean section and avoidance of breast feeding. The use of rapid HIV test may be relevant in Hong Kong because of the large number of unbooked cases with unknown HIV status. The appropriate infection control measures can also be strengthened.
In HIV endemic areas, a second HIV test near term or in labour may be indicated even if the initial HIV test in early pregnancy is negative. The repeat test may also be considered in patients with exposure risk after first screening.
Without interventions, the risk of MTCT varies between 20-40%, depending on factors such as breastfeeding, maternal plasma viral load, clinical status, and the antenatal CD4 T-lymphocyte counts. The maternal plasma viral load is recognised as the strongest predictor of transmission. The use of antiretrovirals and HAART can significantly reduce the viral load and thus the risk of MTCT. A combination of interventions (including combination antiretroviral therapy, caesarean section and avoidance of breastfeeding) is associated with a vertical transmission rate of less than 1-2%. In Hong Kong the Scientific Committee on AIDS (subsequently renamed Scientific Committee on AIDS and STI) has developed guidelines on the prevention of perinatal transmission in 2001, which has been revised in 2007 (see Box 35.2).7

Knowing that about 70% of newborns born without interventions are not infected, it is hard to, as a rule, justify TOP on the grounds of maternal HIV infection alone.
In the landmark randomised placebo-controlled trial known as the AIDS Clinical Trials Group (ACTG) protocol 076, a three-part regimen of ZDV started as early as 14 weeks of pregnancy successfully reduced a rate of transmission by 68%, from 25.8% to 8.3%.11 The benefit of this intervention, which was rapidly introduced with considerable success in developed countries, has been supported by many observational studies. Therefore, all pregnant women who are HIV positive should be offered antiretroviral therapy to prevent MTCT. The use of antiretrovirals during pregnancy is covered in the next section.
Caesarean section (CS), when performed before the onset of labour and/or membrane rupture, is associated with decreases in MTCT ranging from 55-80% in the absence of antiretroviral therapy and with ZDV monotherapy. Recent data from PACTG 36712 suggested that in all subgroups of viral load, combination antiretroviral therapy was associated with the lowest rates of transmission and with viral load <1000 copies/mL, MTCT rates were significantly lower with multiagent vs single-agent antiretroviral therapy (0.6% vs 2.2%) but did not differ by mode of delivery. For women with detectable plasma viral load and who are not taking multiagent therapy, delivery by elective CS is of clear benefit in reducing the risk of MTCT. However, whether elective CS is of benefit in women taking multiagent therapy who have an undetectable plasma viral load at the time of delivery is uncertain. Current recommendations on the application of CS is summarised in Box 35.3. In practice, elective CS is considered when:
(a) The mother cannot tolerate antiretroviral prophylaxis.
(b) The mother presents late in pregnancy so that no adequate prophylaxis can be given.
(c) An unknown viral load level.
(d) Failure of antiretrovirals to suppress viral load to a low level.
(e) Obstetric indications.

Overall breastfeeding appears to increase the risk of perinatal transmission by 5-20%. With access to clean water and adequate medical infrastructure, the balance of risks in Hong Kong is clearly in favour of formula feeding.
General principles - Antiretroviral therapy is now the standard in the prevention of MTCT. The goals in the use of antiretroviral drugs during pregnancy are two fold: (a) treatment of maternal infection and (b) reduction of the risk of perinatal transmission. Pregnant women meeting the treatment criteria for other adults should be offered standard HAART.
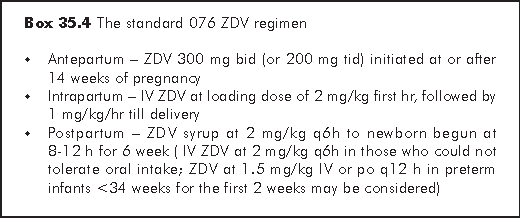
Regimen with ZDV - The ACTG 076 regimen11 is the standard for reference. The three-part ZDV chemoprophylaxis regimen is a minimum (see subsection on HAART below) for all HIV-infected pregnant women to reduce the risk of perinatal HIV transmission (Box 35.4). In women already on HAART when they become pregnant, ZDV should be included as part of regimen after 14 weeks' gestation, if feasible. However, ZDV should not be substitutes for another antiretroviral agent when this is likely to reduce the efficacy of this regimen in treatment of maternal disease, i.e. with previous clinical failure of ZDV or history of documented ZDV resistance.
Although subjects of ACTG 076 were limited to those with CD4 counts above 200/μL, a subsequent trial, ACTG 185, confirmed that the efficacy of ZDV could be extended to those with CD4 below 200/μL. Importantly, in this study subjects previously on ZDV also derived benefit.15 The major concern of using monotherapy in this situation is the development of resistance. In PACTG316, perinatal transmission was low at 1.5% in women on HAART, confirming that the use of HAART with the incorporation of ZDV provides the best effect to prevent MTCT.16
HIV diagnosis during pregnancy - A woman who is diagnosed HIV positive during the antenatal period should receive the same standard of care established for the non-pregnant women. In those with symptomatic disease or a low CD4 count (say, less than 200/μL), ZDV-containing HAART is the preferred regimen. For those not yet clinically indicated for maternal treatment, the same HAART is also the preferred regimen. Other than ZDV, lamivudine (3TC) plus a protease inhibitor are recommended. Of the PIs available, nelfinavir and boosted lopinavir are recommended based on experience and pharmacokinetic data (algorithm). During intrapartum. HAART and intravenous ZDV should be given. Following delivery, the neonate shall continue to receive ZDV for 6 weeks. The treatment of the mother should be advised by the HIV physician.
Regimens in late presentation - Since the ACTG 076 study, there have been many studies of different antiretroviral regimens in different scenarios. Examples are the Thai simplified AZT regimens17 at 36 weeks, HIVNET01218 using 2 doses nevirapine (NVP) at labour and after delivery and dual therapy of AZT/3TC at 36 weeks in PETRA study.19 Overall, in terms of preventing MTCT, a 3-part regimen is better than 2-part, which in turn is better than perinatal prophylaxis only. If a women presents in labour, the preferred intrapartum regimen is:
(a) IV ZDV (see Box 35.4) beginning at onset of labour or three hours before elective CS.
(b) 3TC 150 mg po at onset of labour then 150 mg po q12h till delivery.
(c) NVP single dose 200 mg at onset of labour.
After delivery, the mother should continue ZDV/3TC for 7 days to reduce the development of NNRTI resistance. The baby should continue to receive oral or IV ZDV for 6 weeks (Box 35.4) plus a single dose NVP 2 mg/Kg at 48-72 h. As the field of antiretroviral therapy is changing rapidly, the best regimen shall be developed in consultation with HIV physicians. For women presenting after delivery, there should be immediate administration of ZDV (continued for 6 weeks) and NVP to the neonate. This should be done as soon as possible. Initiation of treatment after 48 h is normally not indicated, and may contribute to viral resistance should infection occur.
The prevention of MTCT does not necessarily equate a healthy baby. The health status of a newborn may suffer on several fronts:
(a) Negative impacts of maternal HIV disease - HIV disease may confer a worsened pregnancy outcome.20 This may be mediated through associated risks such as drug use and poor access to antenatal care as well as the disease itself.
(b) Fetal exposure to antiretrovirals - While potent antiretrovirals may decrease MTCT and control maternal disease, the fetus may suffer adverse effects and possible teratogenicity. With the exception of efavirenz and delavirdine, there is no human evidence of teratogenicity with the current antiretroviral therapy, though data are limited for many of the newer drugs and their combination.
(c) Expert paediatric care - The prevention of MTCT does not stop at delivery. Expert paediatric care has to be provided for optimal continuation and monitoring of antiretrovirals, establishment of HIV diagnosis, initiation of PCP prophylaxis, and the supervision of replacement feeding. Further details of the neonatal and paediatric complications are discussed in the paediatric section.
The quest for preventing the baby from HIV infection should not compromise the welfare of the mother. Potential dilemna may occur in the following circumstances:
(a) continuation or initiation of antiretrovirals for maternal disease in the first trimester of pregnancy,
(b) use of antiretrovirals for prevention of MTCT in a mother whose disease does not require treatment yet,
(c) the potential impacts of elective CS on maternal morbidity, and
(d) avoidance of breast feeding which is important for maternal baby bonding.
These issues are complex and all pros and cons should be fully explained to the mother to allow her to make an informed decision.
A diagnosis of maternal HIV infection carries enormous social and psychological implications, which arise largely as a result of the stigma attached to the infection. The health care provider is faced with the following challenges:
(a) encouraging partner referral for HIV testing,
(b) preparing the mother for the dual challenge of preventing transmission to the baby and taking care of her own health,
(c) enlisting support from social service agencies, this is especially important for those non-Chinese mother/couples where social support is usually weak,
(d) creating a supportive environment for the newborn baby, which poses a challenge also to the sick mother, and
(e) the need to accomplish the above in a relatively short time.
Today, the science of HIV treatment and prevention against MTCT is complicated and advancing rapidly. To ensure that the best management is offered, negative psychosocial factors in the patients and staff should be handled with tact and sensitivity. This may turn out to be the most difficult of all.
1. Anderson JR (ed). A Guide to the Clinical Care of Women with HIV/AIDS, 2005 edition. Rockville:DHHS, 2005. Available from http://hab.hrsa.gov/publications/womencare05 (accessed 16 December 2006).
Committee on Promoting Acceptance of People Living with HIV/AIDS (CPA) of the Hong Kong Advisory Council on AIDS. Recommended Ethical Principles Regarding the Use of Assisted Reproduction in HIV Infected Individuals, 2004.
Dunn DT, Brankt CD, Krivine A, et al. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contribution of intra-uterine and intra-partum transmission. AIDS 1995;9:F7-11.
Landesman SH, Kalish LA, Burns DN, et al. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. The Women and Infants Transmission Study. N Engl J Med 1996;334:1617-23.
Chuachoowong R, Shaffer N, Siriwasin W, et al. Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. Bangkok Collaborative Perinatal HIV Transmission Study Group. J Infect Dis 2000;181:99-106.
Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 2000;283:1167-74.
Tse HY, Lai FK, Wong J, Chan AS, Tang LC. Universal screening of human immunodeficiency virus infection in pregnant women in Hong Kong: prospective study. Hong Kong Med J 2001;7:246-50.
Scientific Committee on AIDS and STI. Recommended clinical guidelines on the prevention of perinatal HIV transmission. Hong Kong: Centre for Health Protection, 2007.
Hong Kong College of Obstetricians and Gynaecologists. Guideline for HIV testing in pregnancy, November 2001.
Scientific Committee of the Advisory Council on AIDS, Hong Kong. Report on the implementation of the universal antenatal HIV testing programme in the public services, 2003.
Scientific Committee on AIDS co-sponsored by the Hong Kong Advisory Council on AIDS and the Centre for Health Protection, Department of Health. Evaluation of effectiveness and efficiency of Universal Antenatal HIV testing programme in Hong Kong-review of the year 2001 to 2004, 2005.
Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994;331:1173-80.
Shapiro D, Tuomala R, Pollack H, et al. Mother-to-child HIV transmission risk according to antiretroviral therapy, mode of delivery, and viral load in 2895 U.S. women (PACTG 367). 11th Conference on Retroviruses and Opportunistic Infections; Feb 8-11 2004; San Francisco, CA. Abstract 99.
Perinatal HIV Guidelines Working Group. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. 12 October 2006. Available from http://www.aidsinfo.nih.gov/guidelines (accessed October 15, 2006).
ACOG. Scheduled Cesarean delivery and the prevention of vertical transmission of HIV infection. ACOG Committee Opinion #234, May 2000.
Stiehm ER, Lambert JS, Mofenson LM, et al. Efficacy of zidovudine and human immunodeficiency virus (HIV) hyperimmune immunoglobulin for reducing perinatal HIV transmission from HIV-infected women with advanced disease: results of Pediatric AIDS Clinical Trials Group protocol 185. J Infect Dis 1999;179:567-75.
Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA 2002;288:189-98.
Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet 1999;353:773-80.
Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795-802.
Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet 2002;359:1178-86.
Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol 1998;105:836-48.