Susan S CHIU
In Hong Kong, the number of known human immunodeficiency virus (HIV) infections in children has remained small. A majority of the infected children in the mid- and late-eighties were haemophiliacs or recipients of contaminated blood. Mother-to-child transmission is now the single most important mode of HIV infection in children in Hong Kong. Early identification of HIV-infected pregnant women prior to delivery could effectively reduce perinatal transmission of HIV infection. The proportion of infections occurring in utero is estimated to be approximately 25% to 40% among children who are not breast-fed.1 Mathematical modelling suggests that much of this in utero transmission occurs relatively late in gestation.2 The absolute risk for in utero transmission is estimated to be approximately 5% or 6%, and for intrapartum transmission, approximately 13% to 18%. Since the introduction of universal antenatal screening for HIV in Hong Kong in 2001, only one infected infant has been born to date to a woman as a result of late presentation (as of the end of 2006).
Advances in HIV treatment are changing the landscape of HIV/AIDS in the clinical setting. Prior to effective viral suppressive therapy, a majority of the infected infants developed marked immunosuppression and AIDS-defining conditions by approximately seven years of age. Pneumocystis jiroveci pneumonia (PCP), HIV encephalopathy, developmental delay, failure to thrive and recurrent bacterial infections were commonly observed problems. Today, effective highly active antiretroviral therapy (HAART) makes it possible for children in Hong Kong to experience prolonged viral suppression and live essentially free of opportunistic infections.3
This chapter summarises a standard approach in the management of infants and children infected with HIV, based on scientific research and international experience and recommendations.4-7 However, since research in children usually lags behind that in adults, some recommendations are extrapolated from adult data.
The guiding principles in the following recommendations are:
(a) HIV-exposed children should be evaluated as soon as possible after birth for the diagnosis of HIV infection.
(b) Postnatal antiretroviral treatment should be completed according to the perinatal prophylaxis regimen chosen for MTCT prevention.
(c) Prophylaxis against PCP should be commenced at 6 weeks of age for all infants born to an HIV-infected mother.
(d) Early treatment of HIV-infected infants regardless of clinical and immunologic parameters is the preferred approach for achieving viral suppression.
(e) Childhood immunisation is an important part of the management programme for HIV infected children, the practice of which is similar to that for healthy infants and children with slight adjustment.
(f) HAART should be used in the management of HIV-infected children.
(g) A multispecialty, multidisciplinary approach involving the following expertise is needed for the comprehensive care of HIV-infected children: paediatric infectious disease, paediatric neurology, paediatric cardiology, nursing, social work, psychology, nutrition, and pharmacology. Life-long continuous care is recommended.
(h) Recommendations for therapy and management will have to be updated frequently as the management of HIV infection in infants, children and adolescents is rapidly evolving and becoming increasingly complex.
(i) A mechanism should be in place to enhance the local knowledge base in HIV management in children, and the exposed children (infection or otherwise) to antiretroviral treatment.
(j) HIV- and antiretroviral-exposed but uninfected children should be followed.
A child born to an HIV-infected mother is considered uninfected if there is no clinical evidence of HIV infection, and in the absence of breastfeeding, (a) 2 or more virologic determinations (culture or PCR) are persistently negative, both of which are performed at 1 month of age or older, and one of which is performed at 4 months of age or older, or (b) 2 negative HIV antibody (IgG) tests at least 1 month apart, performed at greater than 6 months of age.4
Previous international guidelines recommended DNA PCR as the virologic method for early diagnosis of HIV infection in infants. Currently, HIV RNA PCR seems to be as sensitive as HIV DNA PCR for diagnosis in infants. Several studies showed sensitivities of 25-40% in the first week of life, increasing to 90-100% by 2 to 3 months of age.8-11 Testing should be performed within the first 48 hours of life. Additional testing at 14 days of life should be considered since it may lead to early detection of infection. Infants with 2 positive results can be determined to be infected.
Infants with negative tests during the first 6 weeks of life should be re-tested between 1 to 2 months of age, and again between 3 to 6 months of life.4 Initially there was concern that prophylactic antiretroviral treatment of the infant could interfere with the early diagnosis of HIV infection. However, to date, zidovudine (ZDV) has not been shown to decrease the sensitivity and predictive values of virologic assays. Whether HAART regimens that some pregnant women receive for their own HIV disease will affect the diagnostic sensitivity in their infants is unknown.5
Documentation of loss of maternal HIV antibodies between 12 and 18 months is recommended to definitively confirm the absence of HIV infection in a child born to an HIV infected mother. A positive HIV antibody after 18 months of age indicates HIV infection.
All infants born to HIV-infected women who have been started on a ZDV containing regimen should receive a 6-week course of oral ZDV. ZDV is also recommended for the infant born to an HIV-infected woman who has received no antiretroviral therapy during pregnancy or delivery, either alone or in combination with other antiretroviral drugs like lamivudine (3TC) or nevirapine (NVP).7 If the mother is not diagnosed until labour and has received an alternative antiretroviral prophylaxis regimen (ZDV-3TC combination or a single dose of NVP), the infant should receive the same antiretroviral agent according to the regimen. Women who have known ZDV resistance should still receive intravenous ZDV during labor, along with their antiretroviral regimen, and their infants should receive oral ZDV according to the PACTG 076 protocol. However, many clinicians would choose to give additional antiretroviral agents to the infant in combination with ZDV. The optimal prophylactic regimen for such newborns is unknown (refer to Chapter 35).
Breastfeeding by HIV-infected mothers is contraindicated in Hong Kong since there are safe alternatives to breast milk.
Complete blood picture (CBP) and differential count should be performed on the newborn as a baseline evaluation before administration of ZDV. Anaemia is the primary short-term complication of the 6-week ZDV regimen in the neonate. Repeat measurement of haemoglobin is required during and after the completion of the regimen. Infants who have anaemia at birth or who are premature warrant more intensive monitoring.
CD4+ lymphoctye count and percentage should be monitored at 1 and 3 months of age and then continued at 3-month-interval until HIV infection in the infant can be ruled out.
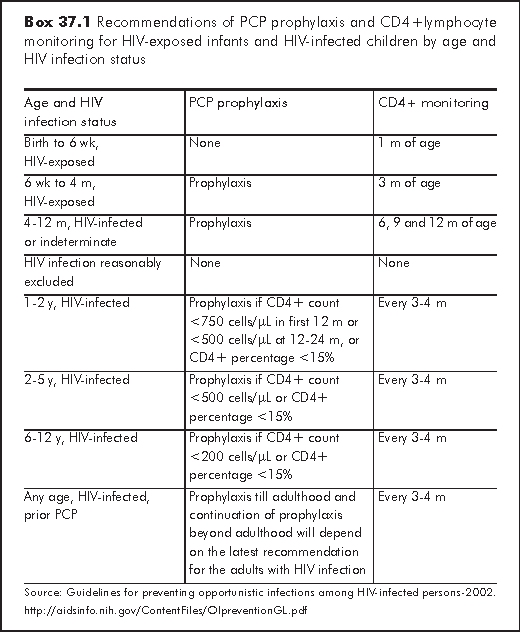
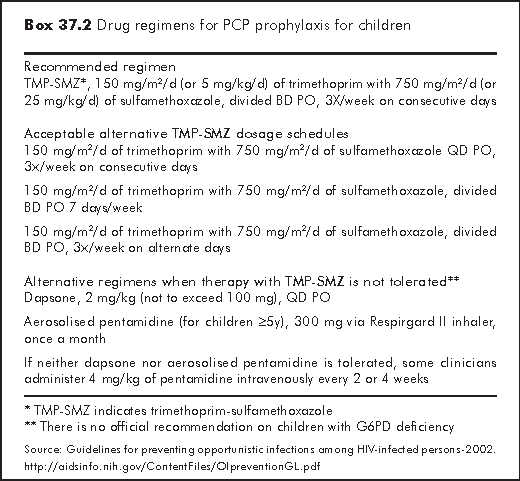
PCP used to be the most common AIDS presenting illness in children before the days of effective antiretroviral therapy. It occurs most often between 3 and 6 months of age when many HIV-exposed infants have not yet been identified as being infected. In 1995, CDC revised the recommendation for PCP prophylaxis in HIV-exposed infants.11 All infants born to HIV-infected women should begin prophylaxis at 4-6 weeks of age, regardless of CD4+ lymphocyte counts or percentage (Box 37.1).4 Newer guideline recommends starting at 6 weeks of age upon the completion of ZDV prophylaxis.7 The drug regimens are shown in Box 37.2.
Prophylaxis should be discontinued for children who are determined not to be infected with HIV. While primary PCP prophylaxis is recommended to be discontinued in adults and adolescents who respond to HAART with a sustained increase of CD4+T cell count to >200 cells/μL for >=3 months, the safety of such practice among HIV infected children on HAART has not been studied extensively. However, recent accumulating paediatric data suggest that discontinuing primary prophylaxis is safe when CD4+ T cell count increases above the age-related CDC thresholds for 3 months.12-14 If primary prophylaxis is discontinued, prophylaxis should be reinitiated if the CD4+ T cell count or percentage decreases to below age-appropriate CDC threshold. Children who have had PCP infection should continue lifelong secondary prophylaxis until more data are available.

The following recommendations apply to all newly diagnosed HIV infected children. Antiretroviral therapy is covered in the next section.
Depending on the individual situation, baseline assessment of the child and family may be done in the in-patient setting. This approach has several advantages: more time can be spent during the initial encounter between the family and the medical team; the family can familiarise themselves with the medical team and the hospital; doctors and nurses can observe the social dynamics of the family; and the initiation of antiretroviral drugs can be supervised and monitored.
Attention should be paid to clinical symptoms commonly seen in HIV infection, e.g., failure to thrive, developmental delay, lymphadenopathy, hepatomegaly, splenomegaly, candidiasis. The clinical categorisation of paediatric HIV infection is detailed in Box 37.3. If the child is the first member of the family diagnosed with HIV infection, both parents and other siblings should also be counselled and evaluated for HIV infection.


The main investigations are T-cell subset enumeration and viral load measurement. Other investigations are also included in this section.
(a) T-cell subsets
CD4+ lymphocyte absolute number and percentage are surrogate markers of disease progression in HIV infection and should be monitored every 3 months. Monitoring should be performed more frequently if there is clinical or immunologic deterioration. Profound decrease in CD4+ lymphocyte counts in the first year of life signifies rapid progression of HIV disease and indicates the immediate need for HAART. Infected infants who have a thymic defect lymphocyte immunophenotypic profile (CD4+ T cell count <1,900/μL and CD8+ T cell count <=850/μL) during the first 6 months of life also have a more rapid HIV disease progression.15 The immunological classification system for HIV infection in children is in Box 37.4.
(b) HIV virus load
Quantification of free virus in plasma can be performed using HIV RNA assays. The dynamics of HIV RNA burden observed in infants is very different from that of adults. Perinatally infected infants exhibit primary HIV viraemia in the first month of life and they have a relatively immature system. They exhibit an extremely high plasma virus load, commonly greater than 106 copies/mL plasma by HIV RNA PCR. Over time the virus load tends to decrease. In general, elevated HIV RNA viral load after the first month of life correlates with rapid disease progression, especially if CD4+ T cells percentage is <15%, although some children with high HIV RNA levels in the first year do not progress as rapidly.16 Due to considerable intrapatient biologic variability in HIV RNA levels, only changes greater than 0.7 log in HIV RNA viral load in children under 2 years and those greater than 0.5 log in children older than 2 years should be considered significant. HIV RNA should be measured every 3 to 4 months in an infected child, and should be monitored with increased frequency if there is virologic or clinical deterioration or when there is a change of antiretroviral therapy.
(c) HIV resistance testing
Testing for resistance should be considered prior to initiation of therapy in newly diagnosed infants under 12 months of age, particularly if the mother is known or suspected to carry drug-resistant virus. However, there are no data to show that resistance testing in this setting improves initial treatment success. Resistance testing should be performed when a change of antiretroviral therapy regimen is being considered for a child who has virologic failure.
(d) Other laboratory investigations
Baseline assessment includes CBP with differentials, liver and renal function tests, lipid, amylase, lipase levels, lactic dehydrogenase and quantitative immunoglobulins. Baseline antibody titres should be considered for toxoplasma, cytomegalovirus (CMV), Epstein-Barr virus, varicella zoster virus, herpes simplex virus (HSV) and hepatitis viruses. Initial titres drawn at the neonatal period would reflect the immune status of the mother. Repeat testing should be done at 12 months of age and then annually if they are negative. The results provide information about these children's exposure and susceptibility to specific infection. For example, CMV negative HIV infected children should receive CMV negative blood in the case of transfusion and if unavailable, leukofiltered blood should be used if possible. For older children, functional antibodies against common antigens could be assessed. This is usually achieved by measuring their immune status after routine immunisation, e.g. IgG against measles and tetanus. Since primary CMV infection in the first month of life has been associated with an increase in HIV replication, urine culture for CMV may also be obtained in the first 6 months of age.
(e) Other evaluations
(i) Chest X-ray - A baseline chest X-ray should be obtained and then annually even in asymptomatic children. This test identifies mediastinal enlargement, lung lesions, lymphoid interstitial pneumonitis (LIP) and cardiomegaly. Patient with chronic lung changes should also have oxygen saturation measured at every visit.
(ii) Cardiac assessment - HIV cardiomyopathy starts early in life. When patients are examined by ECG or during autopsy, cardiac abnormalities are detected more often than expected from physical examination. Subclinical cardiac abnormalities are common, which may be persistent and progressive. A baseline and annual cardiac assessment that includes at least a CXR and an ECG is recommended.
(iii) Visual screening - Children who can co-operate with the examiner should have an annual ophthalmology examination. Children with immune category 3 should preferably be examined by an ophthalmologist every 6 months, especially if they are seropositive for toxoplasmosis or CMV.
(iv) Neurodevelopmental assessment - For older children, as well as young infants with neurologic deficits, imaging of the brain (MRI or CT) should be performed at baseline for evaluation of possible brain atrophy. Older children are referred for a baseline neurodevelopmental assessment by a neurologist. Infants can be referred after 6 months of age if there are no neurologic symptoms, and earlier if they are symptomatic.
(v) Psychosocial assessment - The diagnosis of HIV infection in a child is very devastating for a family. Since the care of an HIV-infected child is a chronic issue, clinicians should aim to establish a long-term relationship with the patient and the family. The establishment of trust and rapport greatly improves adherence to medical treatment. The family should be assessed by the medical social worker for addressing their social service or financial support. Members of the family should be offered referral to the clinical psychologist. Referral to non-government organisations in the field of HIV care should also be considered. These organisations provide invaluable integrated services including home care nursing, physiotherapy, counselling and activities to families of HIV-infected individuals.
Caregivers should be advised to avoid consumption of raw or undercooked meat, seafood or poultry, unpasteurised milk products as well as food prepared under doubtful hygiene conditions to decrease the risk of enteric infection. They should also be advised of the potential risks of infection from pets, e.g. cats that can transmit toxoplasma and bartonella, and turtles and reptiles that can transmit salmonella. Exposure to young farm animals should also be avoided to reduce the risk of cryptosporidiosis. HIV infected children should not drink or swim in lake or river water to reduce the risk of Cryptosporidium or Giardia infection. Practice of good handwashing and personal hygiene should be emphasised.
For specific infections, for example, PCP, prophylaxis is considered (covered in the previous section). HIV-exposed and infected infants and children should receive standard paediatric immunisations with a few exceptions. Inactivated polio vaccine (IPV) should be given instead of the live oral polio vaccine (OPV). Measles-mumps-rubella (MMR) vaccination is recommended for HIV-exposed and HIV-infected children who are not severely immunocompromised (CDC immune category 3). This is due to the concern of possible dissemination of live attenuated vaccine viruses. Varicella vaccine should be given to infected children who are asymptomatic and not immunocompromised (i.e., in immune category 1). (Guidelines for preventing opportunistic infections among HIV-infected persons-2002. http://aidsinfo.nih.gov/ContentFiles/OIpreventionGL.pdf) The latest recommendation for varicella vaccination is that 2 doses are needed. Influenza vaccine should be given seasonally and repeated annually for children who are at least 6 months of age and are infected, or living with persons who are infected, with HIV. Conjugate Haemophilus influenzae type b vaccine and conjugate pneumococcal vaccine should be given according to schedule starting at 2 months of age. In countries where the prevalence of tuberculosis is high, the World Health Organization recommends that BCG vaccination for infants at birth should be a standard practice and this applies to asymptomatic HIV infected infants on a risk-benefit basis. BCG is therefore recommended to all infants born to HIV-infected mothers in Hong Kong.
Breastfeeding by an HIV-infected mother carries a 16% excess risk of HIV infection for the infant and should be avoided. Wasting syndrome is a significant problem in HIV infected children, accounting for 17% of the reported AIDS-defining condition in the US in 1994. HIV-infected children require high-energy, high-protein, nutrient-dense diets.6,17 For early intervention, all infants and children diagnosed of HIV infection should receive a baseline nutritional assessment within 3 months of diagnosis with follow-up every 1 to 6 months depending on the child's status and condition.
Studies suggest that children who know their HIV status have higher self-esteem than children who are not aware of their diagnosis, and parents who have disclosed to their children experience less depression than those who do not. There is no arbitrary age of disclosure but the American Academy of Pediatrics recommends disclosure to school age children.18 However, concrete guidelines are not available.19 The appropriate time should be determined jointly with the parents and when the child is deemed mature enough. Ongoing support should be provided to the parents and children before, during and after the disclosure is made, since this is an ongoing process as the cognitive and emotional awareness about the impact of the disease develops and evolves as these children enter into different stages of life.
Since drug resistance and virologic failure may be inevitable if viral replication persists in the face of therapy, the immediate goal of therapy should be complete viral suppression. With the implementation of universal testing of pregnant women for HIV infection, most HIV infants can be diagnosed in the first month of life. Provided that the child carers are committed to adherence to long-term therapy, early treatment of these recently infected infants offers the best chance for complete viral suppression. Initiating therapy very early in primary infection may also prevent the spread of HIV to long-lived reservoirs like memory CD4+ T lymphocytes. The goal of antiretroviral therapy therefore includes:
(a) Life prolongation
(b) Prevention of disease progression
(c) Maintenance or improvement of quality of life.
The following are the recommended principles in the use of antiretroviral treatment in infants and children, and the rationale involved:
(a) Early treatment of all infants <12 months of age diagnosed of HIV infection should be considered.
(b) All children >=12 months with clinical AIDS (Clinical Category C) or severe immune suppression (Immune category 3) should be treated.
(c) Treatment should be considered for children >=12 months with mild to moderate clinical symptoms (Clinical categories A or B) or moderate immune suppression (Immune Category 2) and/or plasma HIV RNA levels >=100,000 copies/mL.
(d) Many experts would defer treatment of asymptomatic children >=12 months with normal immune status, viral load that is not high (e.g., HIV RNA <100,000 copies/mL) and factors like drug safety and concern for adherence due to unreliable caregivers exist.
(e) The regimen should be effective in achieving a sustained viral suppression and the side effects should be tolerable.
(f) HAART is indicated.
When antiretroviral treatment is indicated, HAART should be prescribed. The treatment should only be given by paediatricians experienced in HIV care. The antiretroviral regimen for infected infants of mother who have received antiretroviral treatment should not be routinely chosen on the basis of maternal regimen. However, genotypic mutations conferring resistance have been documented in US infants with perinatal antiretroviral exposure.20 Resistance testing prior to initiation of therapy in infected infants <12 months of age should be considered, although there are no definitive data to support that such practice is associated with greater success of therapy.
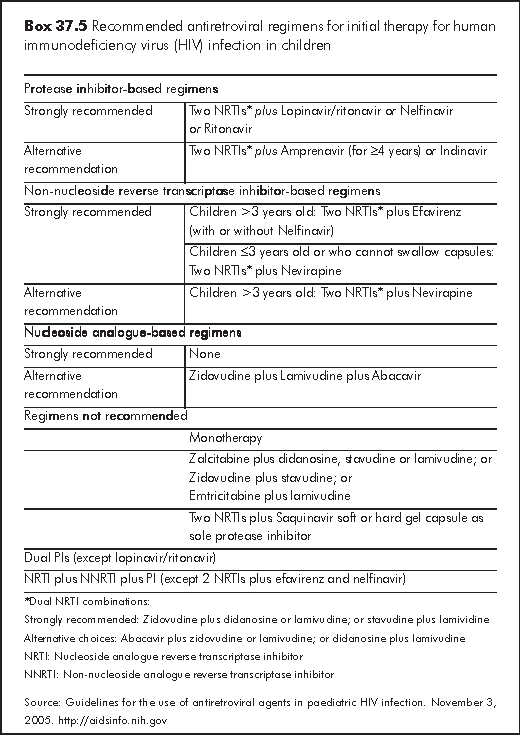
While waiting for more clinical trials of antiretroviral drugs on children, some information regarding the efficacy of these drugs can be extrapolated from trials involving adults. The absence of clinical trials addressing paediatric-specific manifestations of HIV infection does not preclude the use of any approved antiretroviral drug in children. All antiretroviral drugs approved for treatment of HIV infection may be used for children when indicated - irrespective of labelling notations. The recommended regimen includes 2 nucleoside reverse transcriptase inhibitors (NRTIs) and 1 protease inhibitor (PI). The rationale for the choice is to attain maximal suppression of virus replication (Box 37.5). This approach has been successful in children with reduction of HIV RNA to undetectable levels.
For clinicians and patients who prefer to spare the use of protease inhibitors, an option is to initiate therapy with 2 NRTIs with a non-nucleoside analogue reverse transcriptase inhibitor (NNRTI), e.g., efavirenz (EFV) for children over 3 years of age and able to take capsules or NVP for children under 3 years of age. However, the concern for using an NNRTI-based regimen is that there is a low genetic barrier to resistance with NNRTI that will rapidly lead to the development of resistance when therapy does not fully suppress replication.
Since the selection for resistant virus by non-adherence may be worse than ongoing replication of untreated wild type virus, the caregivers need to have a good understanding of the importance of adherence before initiating therapy. It is usually a struggle to administer medications to young children and it is not uncommon that they spit out unpalatable medications. Caregivers should be advised to contact the clinicians immediately if the child repeatedly vomits the antiretroviral drugs. It must be emphasised that the effectiveness of an antiretroviral regimen is directly related to adherence.
Antiretroviral agents are better tolerated by children when compared to adults. Over the past 5 to 10 years, long-term HAART has been shown to be associated with potentially serious metabolic complications such as lipodystrophy, insulin resistance and dyslipidaemia in adults. There are few reports documenting these complications in HIV-infected children. Lipodystrophy syndrome is characterised by peripheral fat wasting, central fat accumulation and metabolic changes. There is no consensus on case definition of HIV-associated lipodystrophy, although two types are described. Lipoatrophy is characterised by localised fat loss in limbs, face and buttocks. Lipohypertrophy, is characterised by central fat accumulation in abdomen, breasts in women and in the posterior neck (buffalo hump). The diagnosis is not easy in children with changing adiposity with normal physiologic growth; estimates of its prevalence range from 1-43%. Protease inhibitors are implicated in the development of lipodystrophy but newer data found the use of d4T (stavudine) and ddI (didanosine) to be associated with an increased risk.21,22 Fasting blood glucose, lipid profile, blood gas and urine glucose should be monitored every 3-4 months.
There are theoretical concerns of potential carcinogenicity of the nucleoside analogue antiretroviral drugs used in children postnatally and/or in utero exposure of ZDV or other antiretroviral agents. NRTIs may inhibit DNA polymerase gamma, a specific mitochondrial enzyme that controls mitochondrial DNA replication. In vitro, the NRTIs have demonstrated significant mitochondrial toxicity, with the eventual development of myopathy. ZDV use during pregnancy has also been associated with the development of mitochondrial toxicity among newborns. The concern for this and other yet unknown potential adverse effects have led clinicians to propose that children with antiretroviral exposure should be followed up into adulthood.15 Long-term follow-up should include annual physical examination and for older adolescent females, gynaecologic evaluation with pap smears has been proposed by some experts. The establishment of a registry to follow up and provide long-term evaluation to antiretroviral exposed children in Hong Kong should be considered.
Fowler MG, Simonds RJ, Roongpisuthipong A. Update on perinatal HIV transmission. Pediatr Clin North Am 2000;47:21-38.
Rouzioux C, Costagliola D, Burgard M, et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. Am J Epidemiol 1995;142:1330-7.
Chiu SS, Lau YL. Update on perinatally acquired human immunodeficiency virus infection in children followed at one centre in Hong Kong. HK J Paediatr (new series) 2006;11:215-22.
Evaluation and medical treatment of the HIV-exposed infant. American Academy of Pediatrics. Committee on Pediatric AIDS. Pediatrics 1997;99:909-17.
Guidelines for the use of antiretroviral agents in pediatric HIV infection. November 3, 2005. Available from http://aidsinfo.nih.gov
Laufer M, Scott GB. Medical management of HIV disease in children. Pediatr Clin North Am 2000;47:127-53.
Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. Available from http://AIDSinfo.nih.gov July 6, 2006.
Delamare C, Burgard M, Mayaux MJ, et al. HIV-1 RNA detection in plasma for the diagnosis of infection in neonates. The French Pediatric HIV Infection Study Group. J Acquir Immune Defic Syndr Hum Retrovirol 1997;15:121-5.
Simonds RJ, Brown TM, Thea DM, et al. Sensitivity and specificity of a qualitative RNA detection assay to diagnose HIV infection in young infants. Perinatal AIDS Collaborative Transmission Study. AIDS 1998;12:1545-9.
Cunningham CK, Charbonneau TT, Song K, et al. Comparison of human immunodeficiency virus 1 DNA polymerase chain reaction and qualitative and quantitative RNA polymerase chain reaction in human immunodeficiency virus 1-exposed infants. Pediatr Infect Dis J 1999;18:30-5.
1995 revised guidelines for prophylaxis against Pneumocystis carinii pneumonia for children infected with or perinatally exposed to human immunodeficiency virus. National Pediatric and Family HIV Resource Center and National Center for Infectious Diseases, Centers for Disease Control and Prevention. MMWR Recomm Rep 1995;44(RR-4):1-11.
Nachman S, Gona P, Dankner W, et al. The rate of serious bacterial infections among HIV-infected children with immune reconstitution who have discontinued opportunistic infection prophylaxis. Pediatrics 2005;115:e488-94.
Urschel S, Schuster T, Dunsch D, Wintergerst U, Hofstetter R, Belohradsky BH. Discontinuation of primary Pneumocystis carinii prophylaxis after reconstitution of CD4 cell counts in HIV-infected children. AIDS 2001;15:1589-91.
Urschel S, Ramos J, Mellado M, et al. Withdrawal of Pneumocystis jirovecii prophylaxis in HIV-infected children under highly active antiretroviral therapy. AIDS 2005;19:2103-8.
Kourtis AP, Ibegbu C, Nahmias AJ, et al. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med 1996;335:1431-6.
Mofenson LM, Korelitz J, Meyer WA 3rd, et al. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis 1997;175:1029-38.
Fields-Gardner C, Fergusson P; American Dietetic Association; Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: nutrition intervention in the care of persons with human immunodeficiency virus infection. J Am Diet Assoc 2004;104:1425-41.
Disclosure of illness status to children and adolescents with HIV infection. American Academy of Pediatrics Committee on Pediatrics AIDS. Pediatrics 1999;103:164-6.
Gerson AC, Joyner M, Fosarelli P, et al.Disclosure of HIV diagnosis to children: when, where, why, and how. J Pediatr Health Care 2001;15:161-7.
Parker MM, Wade N, Lloyd RM Jr, et al. Prevalence of genotypic drug resistance among a cohort of HIV-infected newborns. J Acquir Immune Defic Syndr 2003;32:292-7.
Miller TL, Mawn BE, Orav EJ, et al. The effect of protease inhibitor therapy on growth and body composition in human immunodeficiency virus type 1-infected children. Pediatrics 2001;107:E77.
Hartman K, Verweel G, de Groot R, Hartwig NG. Detection of lipoatrophy in human immunodeficiency virus-1-infected children treated with highly active antiretroviral therapy. Pediatr Infect Dis J 2006;25:427-31.